Good Doctor Animal Experiment | Safety Study of Intracavitary Circulatory Hyperthermic Perfusion Chemotherapy June 2012 Chinese Journal of Clinical Oncology - Vol. 39, No. 22
To observe the effects of different temperatures during cavity circulatory thermal perfusion chemotherapy (CCTP) on the vital signs and abdominal organs of experimental dogs, and to determine the appropriate temperature for CCTP in experimental animals. Method: Dogs were used as experimental animals, and indicators were measured at abdominal cavity temperatures of 41, 42, and 43°C respectively.
Release time:
2025-09-03
Source:

Experimental Study on the Safety of Cavity Circulatory Thermal Perfusion Chemotherapy*
Liu Wenchao, Li Chunbao, Wang Yu, Li Wei
Abstract Objective: To observe the effects of different temperatures during cavity circulatory thermal perfusion chemotherapy (CCTP) on the vital signs and abdominal organs of experimental dogs, and to determine the appropriate temperature for CCTP in experimental animals. Methods: Dogs were used as experimental animals, and indicators were measured at abdominal cavity temperatures of 41, 42, and 43°C. Each group underwent CCTP with cisplatin at a flow rate of 140 mL/min for 60 minutes, repeated once every 2 days for a total of 3 times. Peripheral blood was drawn before and 24 hours after each CCTP session for examination. Two dogs from each group were sacrificed 24 hours and 2 weeks after the third CCTP to observe morphological changes in visceral organs and conduct pathological examinations of the liver, kidneys, and other organs. Results: At 41 and 42°C with a flow rate of 140 mL/min, three CCTP sessions had no significant effect on the dogs' vital signs or liver and kidney function; at 43°C with the same flow rate, three CCTP sessions affected vital signs and liver and kidney function to varying degrees, with pathological damage observed in liver, kidney, spleen, and intestinal tissues. Conclusion: CCTP combined with cisplatin at 42°C and a flow rate below 140 mL/min for three 60-minute sessions is safe and can be used as the treatment temperature for CCTP combined chemotherapy; 43°C under the same conditions damages physiological functions and is not suitable as a treatment temperature for CCTP combined chemotherapy.
Keywords: cavity circulatory thermal perfusion chemotherapy, safety, thermal injury, thermochemotherapy damage
doi:10.3969/j.issn.1000-8179.2012.22.006
Experimental Study on the Safety of Abdominal Cavity Circulatory Thermal Perfusion
Wenchao LIU, Chunbao LI, Yu WANG, Wei LI
Correspondence to: Wenchao LIU; Email: xjcancer@fmmu.edu.cn
Department of Oncology, Xijing Hospital, The Fourth Military Medical University, Xi'an 710032, China
This work was supported by the National Natural Science Foundation of China (No. 30973437)
Abstract Objective: This work investigated the effect of cavity circulatory thermal perfusion (CCTP) on the vital signs and important organs of the experimental animal dogs at different temperatures. The study likewise aimed to determine the optimal temperature for chemotherapy. Methods: Dogs were used as the experimental animal models for CCTP. CCTP was performed with cisplatin using a hyperthermic intraperitoneal treatment system at 41°C, 42°C, and 43°C (one course of clinical treatment). The hepatic and renal functions were detected in preserved blood samples before CCTP and at 24 h after each course of the treatment. The respective morphological and pathological changes of the major abdominal organs were likewise studied at 24 h as well as 2 weeks after the third course of CCTP. Results: The three courses of CCTP with a perfusion rate of 140 mL/min at 41 or 42°C did not have any observable negative effects on the hepatic and renal functions of experimental animals. On the other hand, the three courses of CCTP with a perfusion rate of 140 mL/min at 43°C had a significant negative effect on the hepatic and renal functions, with histopathological injuries in the liver, kidney, spleen, and intestines. Conclusion: CCTP with a perfusion rate of 140 mL/min combined with cisplatin chemotherapy (three times /60 min) was safe and feasible at 42°C, but could damage visceral organs of experimental animals at 43°C.
Keywords: cavity circulatory thermal perfusion; safety; thermal injury; thermochemotherapy damage
Currently, the main treatments for malignant ascites are diuretics and repeated abdominal puncture to remove ascitic fluid, as well as chemotherapy drugs, but the effects are limited. With the development of hyperthermia therapy, tumor hyperthermia is becoming increasingly important in tumor treatment. Cavity circulatory thermal perfusion (CCTP) has shown good results in treating malignant ascites, performed under mild heat and minimally invasive conditions combined with cytotoxic chemotherapy drugs, with safety being a major concern. This study observed the safety of CCTP and its local and systemic effects on dogs, further clarifying the appropriate temperature for CCTP and providing experimental evidence for clinical application of CCTP in treating malignant ascites.
1. Materials and Methods
1.1 Experimental Materials
Experimental equipment: GDPR-2100S cavity circulatory thermal perfusion machine (developed by Xi’an Good Doctor Medical Science and Technology Co., Ltd.), RM6240 multi-channel physiological recorder (Chengdu Instrument Factory), AI-5600 digital thermometer (Xiamen Yudian Automation Co., Ltd.).
Experimental animals and grouping: 18 healthy adult mixed-breed dogs, both male and female, weighing (18±0.5) kg (provided by Tangdu Hospital of the Fourth Military Medical University, No. 12016). Randomly divided into three groups: Group A (41°C saline + cisplatin, 6 dogs), Group B (42°C saline + cisplatin, 6 dogs), Group C (43°C saline + cisplatin, 6 dogs), housed separately.
1.2 Methods
1.2.1 CCTP treatment: Each dog underwent CCTP treatment according to group, each session lasting 60 minutes, repeated every 2 days for 3 times. Anesthesia was induced by intraperitoneal injection of sodium pentobarbital (50 mg/kg). Abdominal puncture needles (12#) were implanted and fixed on both sides of the dog's abdominal cavity, connected respectively to the inflow and outflow tubes of the GDPR-2100S cavity circulatory thermal perfusion machine. High-precision PT100 temperature sensors were implanted beside the inflow and outflow needles, and also implanted in the left and right upper abdomen. Treatment temperatures were set at 41, 42, and 43°C, with flow rate adjusted to 140 mL/min. Initially, single-channel perfusion mode was used to infuse 2500 mL of saline into the abdominal cavity, controlling the inflow temperature at 41, 42, or 43°C, with cisplatin added at 100 mg/kg; then circulation mode was selected to maintain constant temperature circulation treatment for 60 minutes.
1.2.2 Observation content: Inflow and outflow temperatures were read from the thermal perfusion machine's display; temperatures at the left and right upper abdominal puncture points were read from the AI-5600 digital thermometer; infusion flow rate, heart rate, respiration, mean arterial pressure, ascitic fluid floating matter, and color changes of ascitic fluid were monitored. During treatment, temperatures at four measurement points were recorded every 10 minutes. Peripheral blood was drawn before and 24 hours after each treatment session for examination. After three treatment sessions, two dogs from each group were sacrificed at 24 hours and 2 weeks to observe morphological changes in visceral organs. Heart, intestine, liver, spleen, and kidney tissues were collected and fixed in 10% formaldehyde.
Routine paraffin embedding, sectioning, HE staining, and observation of tissue morphological changes under light microscopy.
1.3. Statistical Methods
Data are expressed as x±s. SPSS 12.0 software package was used for data processing. Analysis of variance was applied for multiple sample pairwise comparisons, with P<0.05 considered statistically significant.
2. Results
2.1. Changes in vital signs of experimental animals after CCTP
In Group A, after 1 hour of CCTP at 41°C, the average body temperature of dogs increased by about 0.2°C, heart rate was (120±5) beats/min, respiration was (15±5) breaths/min, and mean arterial pressure was 125 mmHg. In Group B, after 1 hour of CCTP at 42°C, the average body temperature increased by about 0.5°C, heart rate was (135±5) beats/min, respiration was (23±5) breaths/min, and mean arterial pressure was 130 mmHg. In Group C, after 1 hour of CCTP at 43°C, the average body temperature increased by about 1.5°C, heart rate was (140±5) beats/min, respiration was (30±5) breaths/min, and mean arterial pressure was 120 mmHg.
2.2. Changes in liver and kidney function of experimental animals after CCTP
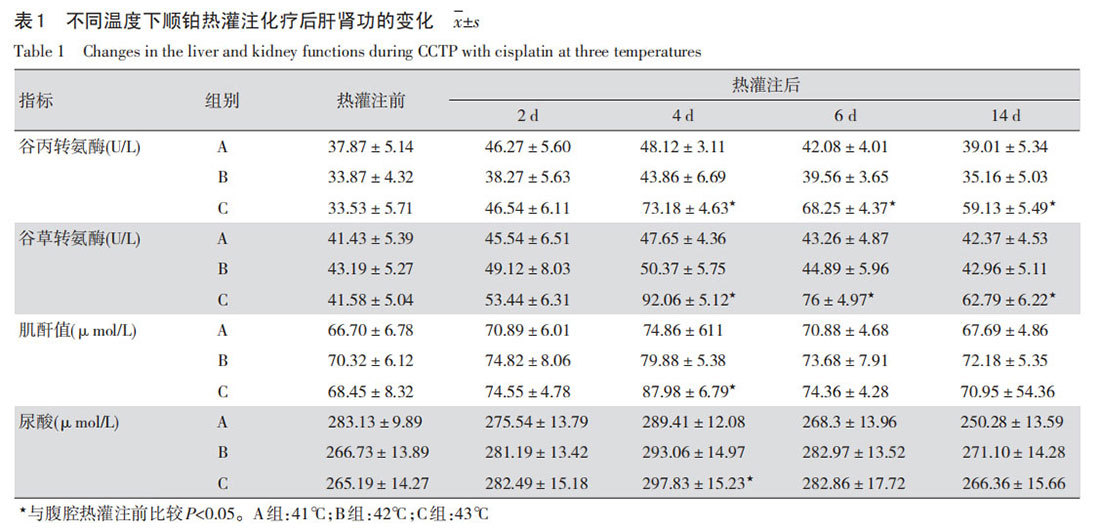
In Groups A and B, at abdominal cavity temperatures of 41°C and 42°C respectively, there were minimal changes in liver and kidney function indicators in dogs. After each perfusion, liver and kidney function indicators showed only slight fluctuations (P>0.05), and basically returned to normal after 2 weeks. In Group C, at an abdominal cavity temperature of 43°C, there was some damage to liver and kidney function in dogs. On day 4, ALT, AST, Cr, and UA values were significantly elevated (P<0.05), and ALT and AST had not returned to normal after 2 weeks (P<0.05, Table 1).
2.3. Morphological changes of abdominal organs in experimental animals after CCTP
In Groups A and B, 24 hours after 3 treatments of CCTP, animals were euthanized and laparotomized. No obvious damage was found in internal organs such as liver, kidney, spleen, and intestines. After 2 weeks, there were no adhesions in the abdominal organs, and no obvious abnormalities were observed macroscopically. In Group C, 24 hours after 3 treatments of CCTP, autopsy revealed adhesions in some areas of the abdominal organs and greater omentum, obvious congestion of mesenteric vessels, small areas of hepatic congestion, purplish-brown bruising and bleeding points in the intestines, no obvious abnormalities in the kidneys macroscopically, and after 2 weeks some intestinal segments still had small bruised areas, but organ adhesions were relieved (Figure 1).
Small bruised areas remained in some intestinal segments, but organ adhesions were relieved (Figure 1).
2.4. Pathological changes of abdominal organs
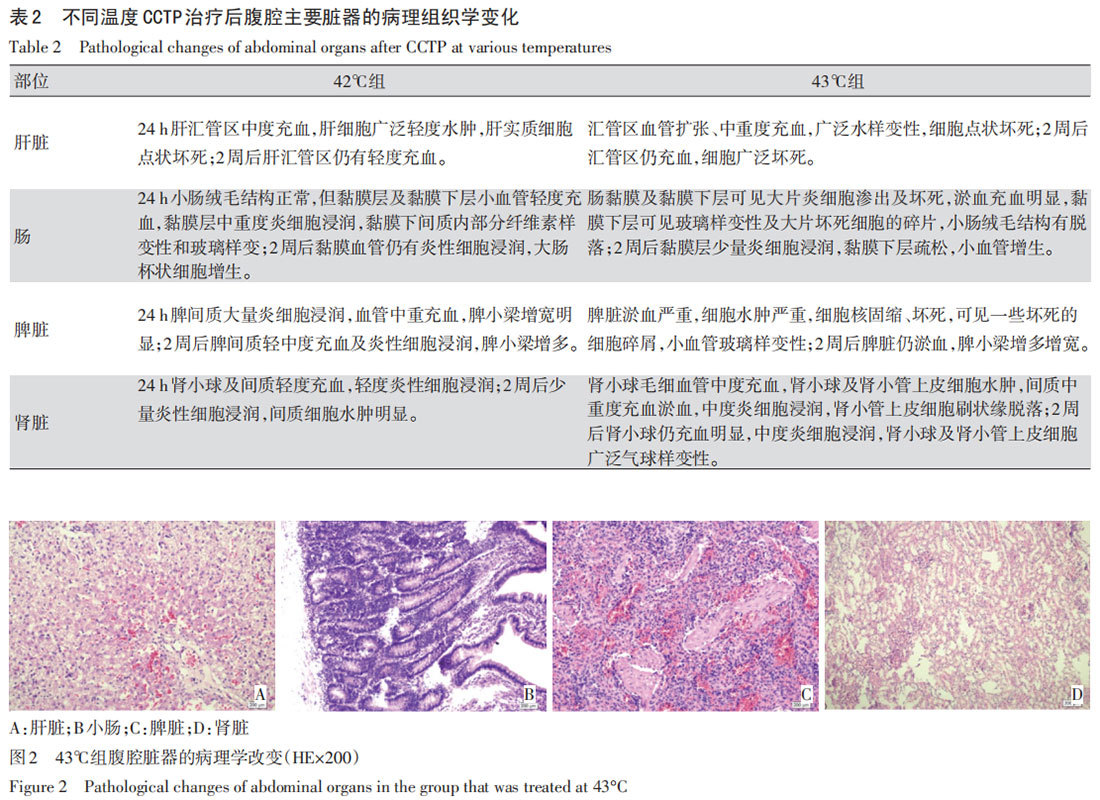
In Group A (41°C), 24 hours after 3 treatments of CCTP, mild congestion of varying degrees was observed in liver, small intestine, kidney, and spleen, with no other changes. Normal conditions were restored after 2 weeks. The main pathological changes in Groups B and C are shown in Table 2 and Figure 2.
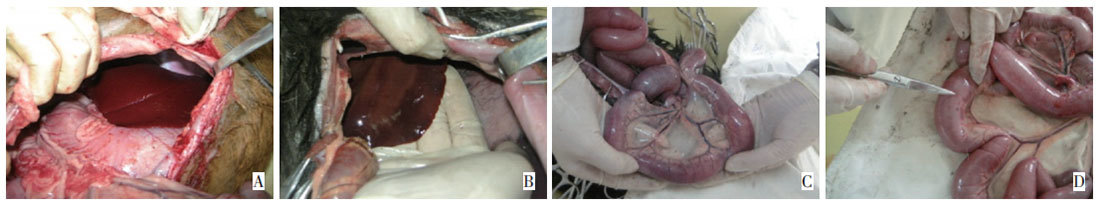
Figure 1 General morphological changes in the abdominal organs 24 hours after three cycles of CCTP at 43°C: small areas of hepatic congestion (A, B), adhesions in some areas of the abdominal organs and greater omentum (C), obvious congestion of mesenteric vessels, purplish-brown bruising and bleeding points in the intestines (D). After 2 weeks, some intestinal segments still had small bruised areas, but organ adhesions were relieved.
Figure 1 General morphological changes in the abdominal organs at 24 h after three cycles of CCTP at 43°C for 60 min. There was extravasated blood in a
small portion of the liver and intestines. The mesenteric vascular vessel was evidently congested, and the omentum adhered to most of the abdominal tissues.
Two weeks after CCTP, some bowel regions were still bruised, but abdominal adhesion was relieved.
3. Discussion
Intraperitoneal metastasis is an important pathway and major cause of death for digestive system tumors, especially gastrointestinal tumors. Intraperitoneal hyperthermic chemotherapy is an important technique in the comprehensive treatment of digestive system tumors.
Body cavity hyperthermic perfusion is divided into extracorporeal radiofrequency abdominal irradiation heating and intraperitoneal circulation heating. The former involves non-flowing perfusate, while the latter involves continuous flowing perfusate. Studies have shown that the relatively safe temperature for non-circulating hyperthermic perfusion is 43°C for 30 minutes. Animal experiments in vitro have also confirmed that the critical damage temperature for various organ tissues is 43°C for 40 minutes [1], but there is a lack of research on the correlation between continuous circulation constant temperature and organ tissue damage. This experiment aims to understand the safe temperature under the combined action of continuous intraperitoneal constant temperature circulation and chemotherapy drug cisplatin.
This study selected dogs as experimental animals to explore the appropriate temperature for intraperitoneal CCTP. Based on animal experiments combined with clinical application requirements, a preliminary experiment was conducted using one dog with simple physiological saline perfusion at 45°C. The result was death on day 3 after three cycles of CCTP at 45°C and flow rate of 140 mL/min. Therefore, it is speculated that the maximum tolerable temperature for dogs undergoing 60 minutes of CCTP with physiological saline plus chemotherapy drugs is around 43°C. After experiments with physiological saline plus cisplatin at flow rates and temperatures of 41, 42, and 43°C, it was concluded that temperatures below 42°C are relatively safe for treatment.
The gross and pathological changes of animal visceral organs in this experiment showed that after three cycles of CCTP (physiological saline plus cisplatin) at an abdominal temperature of 41°C and flow rate of 140 mL/min for 60 minutes, the normal physiological functions of major abdominal organs were not affected, which can be used as a preventive treatment temperature for CCTP. After three cycles of CCTP (physiological saline plus cisplatin) at an abdominal temperature of 42°C and flow rate of 140 mL/min for 60 minutes, mild damage was observed in the liver, kidney, spleen, and intestinal tissues of dogs, and liver and kidney functions were also impaired to varying degrees. However, this acute damage gradually recovered after 2 weeks, indicating reversible injury; thus, it is recommended as a treatment temperature for CCTP. After three cycles of CCTP (physiological saline plus cisplatin) at an abdominal temperature of 43°C and flow rate of 140 mL/min for 60 minutes, severe irreversible damage and necrosis occurred in the spleen, liver, and intestinal tissues of dogs, which did not recover after 2 weeks. Therefore, 43°C is not suitable as a treatment temperature for CCTP.
It is generally believed that the temperature at which the maximum effect on tissue cells is exerted is 42-43°C [2-4]. Temperatures below 40°C have little effect on tissues and organs and show no significant therapeutic effect. When the temperature is 43-45°C, tissues and organs show certain damage, cell lysis, and necrosis, without showing reversal of drug resistance effects [5-6]. Pathological damage to cells at 43°C for 60 minutes is irreversible. Studies have found that the damage mechanism of mild hyperthermia at 41-42°C to tumor tissues includes altering the permeability of tumor cell membranes [7], degrading the extracellular matrix [8], and upregulating the binding ability of cell adhesion molecules [9,13]. In addition, heat can promote the binding of DNA with platinum drugs, increase the intracellular concentration of platinum drugs, and inhibit the repair mechanism of cell damage induced by cisplatin [10,14], i.e., the synergistic effect of chemotherapy drugs and hyperthermia [11]. This experiment (constant temperature circulating hyperthermic chemotherapy perfusion) suggests that the tissue damage temperature is lower than the previously described perfusion temperature [12], possibly related to heating and temperature measurement techniques and other conditions. Secondly, the fluid scouring damage to tissues caused by the circulating perfusion fluid (flow rate 140 mL/min), with greater damage at higher flow rates. Furthermore, the direct damage of chemotherapy drugs to tissues. These results suggest that during circulating hyperthermic perfusion chemotherapy, one should not simply pursue high temperatures (above 43°C) for direct thermal killing of tumors, but also consider the combined damaging effects of perfusion fluid hydrodynamics and drugs.
Currently, the temperature control methods, heating modes, and perfusion patterns of constant temperature circulating hyperthermic perfusion machines used clinically vary, so the thermal damage differs among different models. The GDPR-2100 CCTP machine we use can accurately control and measure temperature, with rapid and uniform abdominal heating, maintaining a constant temperature, ensuring uniform distribution of heat energy and drugs in the body cavity and organs. This study identified three key temperature points: at 41°C with a flow rate of 140 mL/min for 60 minutes, no significant damage changes; at 42°C, reversible mild damage; and at 43°C, more severe and irreversible damage.
Therefore, for experimental dogs, the safe parameters for CCTP are 42°C, flow rate 140 mL/min, abdominal perfusion 3 times, each lasting 60 minutes.
References
1. Li Dingjiu, Wang Yishan. Practical Tumor Hyperthermia [M]. First Edition. Jilin: Jilin Science and Technology Press. 2006:10-14.
2. Agostinelli E, Tempera G, Molinari A, et al. The physiological role of biogenic amines redox reactions in mitochondria. New perspectives in cancer therapy [J]. Amino Acids, 2007, 33(2):175-187.
3. Wartenberg M, Gronczynska S, Bekhite MM, et al. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular prostate tumor spheroids by hyperthermia and reactive oxygen species [J]. Int J Cancer, 2005, 113(2):229-240.
4. Wei Hongmei, Guo Kunyuan, Mei Jiazhuan, et al. Experimental study on the combined effect of hyperthermia and chemotherapy on K562/AO2 cells in vitro [J]. Chinese Journal of Experimental Hematology, 2007, 15(4):724-728.
5. Westermann A, Grosen E, Katschinski D, et al. A pilot study of whole body hyperthermia and carboplatin in platinum-resistant ovarian cancer [J]. Eur J Cancer, 2001, 37(9):1111-1117.
6. Komdeur R, Plaat BEC, Hoekstra HJ, et al. Expression of P-glycoprotein, multidrug resistance-associated protein 1, and lung resistance-related protein in human soft tissue sarcomas before and after hyperthermic isolated limb perfusion with tumor necrosis factor-α and melphalan [J]. Cancer, 2001, 91(10):1940-1948.
7. Sagowski C, Jaehne M, Kehrl W, et al. Tumor oxygenation under combined whole-body hyperthermia and polychemotherapy in a case of recurrent carcinoma of the oral cavity [J]. Eur Arch Otorhinolaryngol, 2002, 259(1):27-31.
8. Fukao H, Ikeda M, Ichikawa T, et al. Effect of hyperthermia on the viability and the fibrinolytic potential of human cancer cell lines [J]. Clin Chim Acta, 2000, 296(1):17-33.
9. Sato T, Sawaji Y, Matsui N, et al. Heat shock suppresses membrane type 1-matrix metalloproteinase production and progelatinase A activation in human fibrosarcoma HT-1080 cells and thereby inhibits cellular invasion [J]. Biochem Biophys Res Commun, 1999, 265(1):189-193.
10. Wiedemann GJ, Robins HI, Katschinski DM, et al. Systemic hyperthermia and ICE chemotherapy for sarcoma patients: rationale and clinical status [J]. Anticancer Res. 1997, 17(4B):2899-2902.
11. Ma Shenglin, Chen Xueqin, Mou Hanzhou, et al. Synergistic killing effect of high temperature combined with cisplatin on human lung adenocarcinoma cell line H1299 [J]. Journal of Clinical Oncology, 2006, 11(6):427-430.
12. Cui Shuzhong, Ba Mingchen, Huang Diwen, et al. Animal experiment on safety evaluation of BR-TRG-I type body cavity hyperthermic perfusion treatment system [J]. Chinese Journal of Comparative Medicine, 2009, 19(10):27-31.
13. Liang H, Li JW, Shi YR, et al. Change in E-cadherin, alpha-, beta- and gamma-catenin expression after hyperthermia of a human colon carcinoma cell line in vitro [J]. Zhonghua Yi Xue Za Zhi, 2004, 84(15):1299-1303.
14. Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia [J]. Crit Rev Oncol Hemat, 2002, 43(1):33-56.
(Received on 2012-10-02)
(Revised on 2012-11-16)
(Article edited by Zheng Li)