Good Doctor Animal Experiment | Animal experiment comparing the flow rate and temperature of intraperitoneal hyperthermic perfusion chemotherapy with three different catheterization methods August 2022 Modern Oncology Medicine - Volume 30, Issue 15
Compare the differences in perfusion flow rate and intraperitoneal temperature between surgical catheter placement and medical peritoneal puncture catheter placement in hyperthermic intraperitoneal chemotherapy (HIPEC) through animal experiments.
Release time:
2025-09-03
Source:
Animal experiment comparing flow velocity and temperature of hyperthermic intraperitoneal chemotherapy with three different catheterization methods
Zhang Tao¹, Li Yan², Liao Chenggong¹, Liu Xiaochen³, Li Xinbao², Yang Xue¹, Li Wei⁴, Liu Lili¹
Animal experiment on the comparison of flow velocity and temperature of hyperthermic intraperitoneal chemotherapy with three different methods
ZHANG Tao1, LI Yan2, LIAO Chenggong1, LIU Xiaochen3, LI Xinbao2, YANG Xue1, LI Wei4, LIU Lili1
1 Department of Oncology, the Second Affiliated Hospital, Air Force Medical University, Shaanxi Xi'an 710038, China; 2 Department of Peritoneal Cancer Surgery, Beijing Shijitan Hospital, Capital Medical University, Beijing 100038, China; 3 Department of Surgical Oncology, Hanzhong 3201 Hospital of Medical College of Xi'an Jiaotong University, Shaanxi Hanzhong 723000, China; 4 Xi'an Good Doctor Medical Science and Technology Co., Ltd., Shaanxi Xi'an 710100, China.
[Abstract] Objective: To compare the difference in flow velocity and temperature between surgical catheterization and abdominal puncture catheterization in hyperthermic intraperitoneal chemotherapy (HIPEC). Methods: Ten pigs were randomly divided into groups A, B, and C. Group A was treated with four-point surgical HIPEC, group B with two-point internal medicine HIPEC, and group C with two-point internal medicine HIPEC for the first 30 minutes and four-point internal medicine HIPEC for the last 30 minutes. Intracavitary thermometers were placed in the left lower abdomen, left upper abdomen, right lower abdomen, and right upper abdomen in all animals, and the initial temperature at each point was recorded. The flow velocity of the perfusion fluid and the temperature at each point were recorded at 15, 30, 45, and 60 minutes of HIPEC, and the vital signs of the experimental animals were monitored during and after the operation. Results: The flow velocity and temperature of the three groups were compared by repeated measures analysis of variance, and there was no significant difference among the three groups (P=0.214, 0.305). There was a significant difference in flow velocity between group A and group C at 15 and 60 minutes (P<0.05), but no significant difference among the three groups at other time points. There was a significant difference in temperature between group A and group B at 45 and 60 minutes, and between group B and group C at 30 minutes (P<0.05). There was no significant difference in temperature among the three groups at other time points (P>0.05). The vital signs of all animals were stable during the operation and recovered well after the operation. Conclusion: Although the drainage tube of internal medicine HIPEC is thinner, two-point internal medicine HIPEC in group B and four-point internal medicine HIPEC in group C can reach the flow velocity and temperature levels of surgical HIPEC in group A, with less trauma and better repeatability, which is worthy of clinical promotion.
[Key words] hyperthermic intraperitoneal chemotherapy, flow velocity, temperature, animal experimentation
Modern Oncology 2022, 30(15): 2680-2684
[Abstract] Objective: To compare the differences in perfusion flow velocity and intracavitary temperature between surgical catheterization and internal medicine abdominal puncture catheterization in hyperthermic intraperitoneal chemotherapy (HIPEC) through animal experiments. Methods: Ten domestic pigs were randomly divided into groups A, B, and C. Group A used four-point surgical HIPEC, group B used two-point internal medicine HIPEC, and group C used two-point internal medicine HIPEC for the first 30 minutes and four-point internal medicine HIPEC for the last 30 minutes. All animals had intracavitary thermometers placed in the left lower abdomen, left upper abdomen, right lower abdomen, and right upper abdomen to record the initial temperature at each point. The flow velocity and temperature of the perfusion fluid were recorded at 15, 30, 45, and 60 minutes of HIPEC, and the vital signs of the experimental animals were monitored during and after the operation. Results: Repeated measures analysis of variance showed no statistically significant differences in flow velocity and temperature among the three groups (P=0.214, 0.305).
Pairwise comparisons of flow velocity and temperature among the three groups showed statistically significant differences in flow velocity between groups A and C at 15 and 60 minutes (P<0.05), with no significant differences at other time points (P>0.05). Temperature differences between groups A and B at 45 and 60 minutes were statistically significant (P<0.05), as were differences between groups B and C at 30 minutes (P<0.05). No significant temperature differences were found among the three groups at other time points (P>0.05). All animals maintained stable vital signs during HIPEC and recovered well postoperatively. Conclusion: Although the drainage tube of internal medicine HIPEC is thinner, two-point internal medicine HIPEC in group B and four-point internal medicine HIPEC in group C can achieve the flow velocity and temperature levels of surgical HIPEC in group A, with less trauma and better repeatability, making it worthy of clinical promotion.
[Keywords] hyperthermic intraperitoneal chemotherapy; flow velocity; temperature; animal experiment
[Chinese Library Classification] R73-3 [Document Identification Code] A DOI:10.3969/j.issn.1672-4992.2022.15.002 [Article Number] 1672-4992-(2022)15-2680-05
Hyperthermic intraperitoneal chemotherapy (HIPEC) refers to the preparation of chemotherapeutic drugs into an intraperitoneal perfusion solution, which is then circulated at a constant temperature through a body cavity hyperthermia perfusion device and retained in the abdominal cavity for a certain period to prevent and treat peritoneal carcinomatosis and malignant ascites caused by it. Due to poor blood supply on the peritoneal surface and the barrier of the plasma-peritoneal membrane, intravenous chemotherapy drugs have difficulty reaching the peritoneum. HIPEC allows high concentrations of chemotherapeutic drugs to directly contact the peritoneum and exerts antitumor effects through the positive mechanical flushing effect of large-volume fluid on the abdominal cavity, the thermal killing effect of high temperature on tumors, and the synergistic effect of chemotherapy and hyperthermia [1]. Therefore, HIPEC has unique efficacy for peritoneal carcinomatosis and malignant ascites caused by various malignant tumors [2-5].
In recent years, many hospitals have introduced HIPEC technology; however, perfusion methods vary among centers, leading to differences in efficacy and adverse reactions. The positive flushing effect during HIPEC largely depends on the flow velocity of the perfusion fluid, while the intracavitary temperature during HIPEC is the key determinant of efficacy. Therefore, comparing the flow velocity and temperature of perfusion fluid in different HIPEC methods is crucial.
Currently, the commonly used HIPEC methods in China mainly include surgical and medical types. Surgical HIPEC often involves placing four drainage tubes during open or laparoscopic surgery, performing HIPEC intraoperatively or postoperatively. These drainage tubes are relatively thick with a high flow rate, quickly reaching the target temperature; however, they require placement under general anesthesia, causing significant trauma, and tubes are usually removed after 1 to 3 HIPEC sessions, making repeated catheterization difficult and limiting repeatability. Medical HIPEC involves ultrasound-guided abdominal puncture to place two or four drainage tubes for HIPEC. This method only requires local infiltration anesthesia, causes less trauma, and allows multiple punctures and catheter placements with good repeatability; however, the drainage tubes are thinner, and there are no reports on whether the perfusion flow rate and intraperitoneal temperature can reach the levels of surgical HIPEC.
Some scholars believe that compared to medical HIPEC, surgical HIPEC has a higher flow rate and more uniform temperature distribution, offering advantages; however, no studies have directly compared the differences in flow rate and temperature between surgical and medical HIPEC. Due to the difficulty of placing multiple temperature sensors in different abdominal locations in patients, we selected domestic pigs, whose abdominal volume is close to humans, as research subjects to compare the differences in perfusion flow rate and temperature between these two HIPEC methods during constant temperature circulation, and to observe whether catheter placement under local anesthesia in medical HIPEC can achieve the flow rate and temperature of surgical HIPEC under general anesthesia, aiming to find a less traumatic and effective perfusion method.
1. Materials and Methods
1.1. Experimental Materials
Experimental animals: Ten mature domestic pigs provided by the Experimental Center of the Second Affiliated Hospital of Air Force Medical University (ear numbers: 161050202595168, 161050202595172, 161050202595198, 161050202595206, 161050202595232, 161050202595233, 161050202595242, 161050202595377, 161050202595923, 161050202595925), weighing 21 to 28 kg, both sexes included. All animals were strictly raised and handled according to the requirements of the Animal Experiment Ethics Committee of the Second Affiliated Hospital of Air Force Medical University.
The GDPR-2100S type body cavity thermal perfusion treatment machine was provided by Xi’an Good Doctor Medical Science and Technology Co., Ltd., with main technical parameters: temperature control accuracy ±0.3°C, flow accuracy ±10%. R620-S1-IECS general animal anesthesia machine: Shenzhen Reword Life Science and Technology Co., Ltd. Umec10 Vet portable multi-parameter veterinary monitor: Shenzhen Mindray Bio-Medical Electronics Co., Ltd. Routine surgical instruments for animal experiments were provided by the Experimental Surgery Department of the Second Affiliated Hospital of Air Force Medical University. Electronic thermometer: AI-5600 handheld high-precision digital thermometer, Xiamen Yudian Automation Technology Co., Ltd. Temperature measurement range: (Pt100 scale) -200 to 850°C, resolution 0.1°C, maximum allowable error ±0.5°C. Cisplatin for injection (lyophilized): 10 mg/vial, Qilu Pharmaceutical Co., Ltd. (National Medicine Standard H20023460), dissolved in 50 mL saline and injected into the perfusion fluid via the instrument's tubing medication port. Saline injection: 0.9% sodium chloride solution, 500 mL/bag, Chenxin Pharmaceutical Co., Ltd., National Medicine Standard H37022337, batch number: 1908330546. Tiletamine hydrochloride and zolazepam hydrochloride injection: brand name Zoletil®50, batch number (2015) Veterinary Drug Certificate No. 43, dosage 0.02 mL/kg, used for animal anesthesia. Chlorpromazine hydrochloride injection: brand name Luminning, produced by Jilin Huamu Health Products Co., Ltd., batch number Veterinary Drug No. (2015) 070011777, dosage 0.01 mL/kg, used for animal anesthesia. An'er iodine skin disinfectant: Shanghai Likang Disinfection High-Tech Co., Ltd. (production batch number: 20190328).
1.2. Methods
1.2.1 Experimental grouping: The experimental animals were randomly divided into groups A, B, and C, with 3 animals in groups A and B each, and 4 animals in group C, each group undergoing different HIPEC methods.
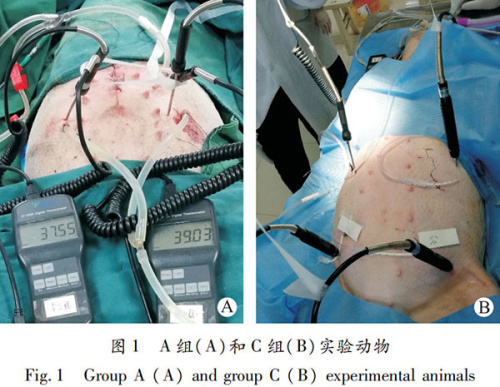
1.2.2 Experimental procedures: Group A used the four-point surgical HIPEC method. After anesthesia, a midline abdominal incision was made, and a 22F drainage tube (diameter 7.3 mm) and an intraperitoneal thermometer were placed in each of the left lower abdomen, left upper abdomen, right lower abdomen, and right upper abdomen. The incision was then sutured. The left and right lower abdomen served as the inflow ports, and the left and right upper abdomen as the outflow ports. Approximately 3,500 mL of 43°C saline was perfused for washing, followed by cisplatin injection into the perfusion fluid at a dose of 2.74 mg/kg, with a target flow rate of 600 mL/min, maintaining continuous constant temperature circulation for 60 minutes (Figure 1A).
Group B used the two-point medical HIPEC method. After anesthesia, ultrasound-guided abdominal puncture was performed to place catheters, with a 12F drainage tube (diameter 4.0 mm) placed in the left lower abdomen and right upper abdomen respectively, and intraperitoneal thermometers placed in the left lower abdomen, left upper abdomen, right lower abdomen, and right upper abdomen. The left lower abdomen served as the inflow port and the right upper abdomen as the outflow port, performing HIPEC using the same method.
Group C used the four-point medical HIPEC method. After anesthesia, ultrasound-guided abdominal puncture was performed to place catheters, with an 8F drainage tube (diameter 2.7 mm) and an intraperitoneal thermometer placed in each of the left lower abdomen, left upper abdomen, right lower abdomen, and right upper abdomen. For the first 30 minutes, the left lower abdomen was the inflow port and the right lower abdomen the outflow port; for the last 30 minutes, the left and right lower abdomen were the inflow ports and the left and right upper abdomen the outflow ports, performing HIPEC using the same method (Figure 1B).
1.2.3 Observation items: The perfusion flow rate was recorded at 15, 30, 45, and 60 minutes during HIPEC. The temperature at each measurement point was recorded at 0, 15, 30, 45, and 60 minutes during HIPEC. Vital signs of the experimental animals were monitored intraoperatively and postoperatively.
1.3. Statistical analysis
SPSS 26.0 software was used for data processing and analysis. Repeated measures ANOVA was used to compare perfusion flow rates and temperatures among the three groups. Pairwise comparisons between groups were performed using the LSD method. The significance level was set at 0.05, with P<0.05 considered statistically significant.
2. Results
2.1. Comparison of perfusion flow rates among the three groups
The perfusion flow rates of the three groups during the HIPEC process were recorded and expressed as x-±s (Table 1), and plotted as line charts (Figure 2). Repeated measures ANOVA showed no statistically significant difference in perfusion flow rates among the three groups (P=0.214). Pairwise comparisons of flow rates between groups showed no statistically significant differences between groups A and B at all time points (t15 min=0.71, t30 min=0.40, t45 min=0.49, t60 min=2.01, all P>0.05); no significant differences between groups B and C at all time points (t15 min=1.98, t30 min=2.21, t45 min=0.44, t60 min=0.43, all P>0.05); significant differences between groups A and C at 15 and 60 minutes (t15 min=2.73, t60 min=2.57, both P<0.05), but no significant differences at 30 and 45 minutes (t30 min=1.78, t45 min=0.96, both P>0.05).
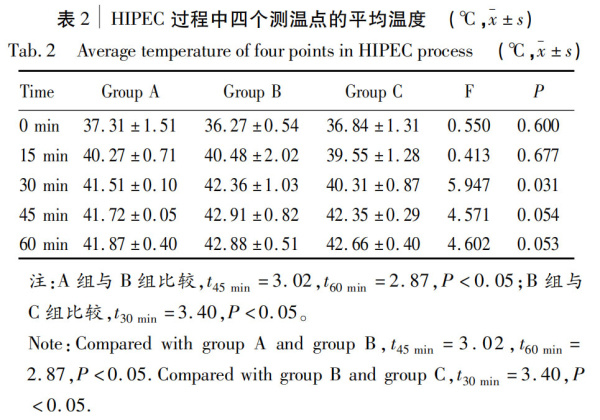
2.2. Comparison of average temperatures among the three groups
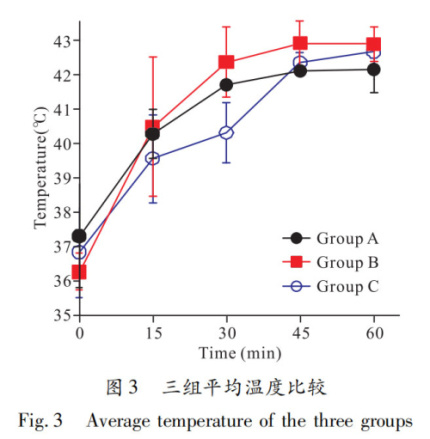
The average temperature at each time point was calculated by averaging the temperatures from four sites on each animal at the same time point, then the average temperature of each group was calculated and expressed as x-±s (Table 2), and plotted as line charts (Figure 3). Repeated measures ANOVA showed no statistically significant difference in average temperatures among the three groups (P=0.305). Pairwise comparisons of average temperatures showed no significant differences between groups A and B at 0, 15, and 30 minutes (t0 min=1.05, t15 min=0.18, t30 min=1.33, all P>0.05), but significant differences at 45 and 60 minutes (t45 min=3.02, t60 min=2.87, both P<0.05); no significant differences between groups B and C at 0, 15, 45, and 60 minutes (t0 min=0.62, t15 min=0.85, t45 min=1.52, t60 min=0.69, all P>0.05), but a significant difference at 30 minutes (t30 min=3.40, P<0.05); no significant differences between groups A and C at any time point (t0 min=0.50, t15 min=0.65, t30 min=1.98, t45 min=1.71, t60 min=2.37, all P>0.05).

2.3. General condition of experimental animals
All animals maintained stable vital signs during the HIPEC process, with body temperature increasing less than 1°C compared to before HIPEC. Heart rate, respiration, and blood pressure remained within normal ranges. Body temperature gradually returned to normal one hour after HIPEC ended. All animals showed good general condition postoperatively, able to eat, drink, and move freely one day after surgery, with good mental status and wound healing, and no obvious adverse reactions.
3. Discussion
Since SPRATT et al. first proposed HIPEC in 1980 [10], scholars at home and abroad have continuously improved HIPEC technology to achieve more stable perfusate temperatures. From simple direct infusion of heated perfusate to thermostatic water baths, endogenous fields, and microwave heating methods, HIPEC technology has been continuously innovated and improved. Currently, HIPEC is widely used in the treatment of peritoneal metastases caused by abdominal malignancies such as ovarian cancer, gastric cancer, colorectal cancer, cholangiocarcinoma, liver cancer, pancreatic cancer, malignant peritoneal mesothelioma, and pseudomyxoma peritonei [2-5].
Surgical HIPEC is often combined with cytoreductive surgery (CRS), used not only for palliative treatment but also for neoadjuvant and adjuvant therapies [11-12]. BONNOT et al. [13] showed that compared with CRS alone, CRS combined with surgical HIPEC can prolong overall survival and recurrence-free survival in patients with gastric cancer peritoneal metastases. FAGOTTI et al. [14] reached similar conclusions in platinum-sensitive recurrent ovarian cancer patients. However, the French PRODIGE 7 study [15] reached an opposite conclusion, comparing CRS combined with surgical HIPEC versus CRS alone in colorectal peritoneal metastases, finding similar survival outcomes between groups. This conclusion has been questioned because the study required only over 30 minutes of HIPEC, and considering oxaliplatin used is a prodrug, 30 minutes may be insufficient. Notably, surgical HIPEC requires 30 minutes to reach a relatively stable temperature, suggesting that 30 minutes of perfusion time may be inadequate.
Current research on medical HIPEC is mostly domestic and limited in number, with no large clinical trials confirming its efficacy. Compared to direct infusion of heated perfusate into the abdominal cavity, medical HIPEC has obvious advantages [16]. Jin Chunying et al. [17] showed that medical HIPEC combined with intravenous chemotherapy can improve malignant ascites in gastric cancer, but there is no direct comparison of efficacy between surgical and medical HIPEC.
The "Expert Consensus on Clinical Application of Intraperitoneal Hyperthermic Chemotherapy Technology in China (2019 Edition)" recommends a HIPEC perfusion flow rate of 400~600 mL/min, perfusate temperature of 43°C, and perfusion time of 60~90 minutes [18]. Existing surgical HIPEC studies mostly meet these standards for flow rate and temperature, but it is unclear whether medical HIPEC can achieve similar perfusion flow rates and intraperitoneal temperatures as surgical HIPEC. Therefore, using domestic pigs as research subjects, we attempted to compare the perfusion flow rates and temperatures during thermostatic circulation between surgical and medical HIPEC, indirectly comparing the efficacy of the two HIPEC methods.
During the first 30 minutes of HIPEC, the perfusion flow rates of groups A and B gradually increased, stabilizing after 30 minutes, both exceeding 550 mL/min, with no significant difference (P>0.05). Since group C performed HIPEC at only two lower abdominal points during the first 30 minutes and used the narrowest drainage tubes, its flow rate was much lower than groups A and B; at 15 minutes, group C's flow rate was only 368 mL/min, significantly lower than group A's 537 mL/min (P=0.029). After adding two upper abdominal points in the latter 30 minutes, group C's flow rate significantly increased; there was no significant difference between groups A and C at 30 and 45 minutes (P=0.118, 0.369), and at 60 minutes, group C's flow rate was even significantly higher than group A's (P=0.037). In summary, the two-point medical HIPEC method in group B can reach the flow rate level of surgical HIPEC in group A, while the two-point medical HIPEC in group C during the first 30 minutes cannot reach the flow rate levels of groups A and B; only after switching to the four-point medical HIPEC in the latter 30 minutes can it reach the flow rate levels of groups A and B.
In the first 30 minutes of HIPEC, the average temperature in groups A and B rose rapidly, stabilizing around 42°C at 30 minutes. At 45 and 60 minutes, the average temperature in group B was significantly higher than in group A (P=0.019, 0.024). Since group C only used two points in the lower abdomen for HIPEC in the first 30 minutes and had the thinnest drainage tubes, the rate of temperature increase was the slowest. At 15 minutes, the average temperature in group C was slightly lower than in groups A and B (P>0.05), but at 30 minutes, group C's average temperature was only 40.31°C, significantly lower than group B's 42.36°C (P=0.011). After adding two points in the upper abdomen in the latter 30 minutes, the average temperature in group C increased significantly, stabilizing above 42°C at 45 and 60 minutes, reaching the levels of the other two groups (P>0.05). These data indicate that the two-point internal medicine HIPEC method in group B can achieve the temperature level of the surgical HIPEC in group A, while the two-point internal medicine HIPEC in group C during the first 30 minutes cannot reach the temperature levels of groups A and B. Only after switching to the four-point internal medicine HIPEC in the latter 30 minutes can group C reach the temperature levels of groups A and B.
It is noteworthy that the average temperature trends in each group corresponded with their flow rate trends, indicating that a certain perfusion flow rate is necessary to maintain intra-abdominal temperature. Overall, group B achieved flow rate and temperature levels comparable to group A. The differing performances of group C in the first and latter 30 minutes suggest that when performing internal medicine HIPEC, using four 8F drainage tubes may be a better choice than two 8F tubes. Therefore, in clinical practice, if 12F drainage tubes are used, the two-point internal medicine HIPEC can reach the flow rate and temperature of surgical HIPEC; if 8F tubes are used, the four-point internal medicine HIPEC is required to achieve the flow rate and temperature of surgical HIPEC and obtain equivalent therapeutic effects.
Regarding the safety of HIPEC, existing studies on surgical HIPEC report adverse reaction rates mostly between 28.8% and 60.0%, mainly including abdominal infections, adhesions, and bleeding [19-20], with mortality rates mostly between 2.9% and 8.3% [4,21-22]. We have studied the safety of internal medicine HIPEC through a retrospective analysis of 670 patients who underwent internal medicine HIPEC 4,249 times in our department. The HIPEC-related mortality rate was 0, and the adverse reaction rate was 2.4%, including perfusion circulation failure (1.3%), puncture site pain (0.5%), intestinal obstruction (0.1%), peritonitis (0.1%), and intestinal perforation (0.07%) [23].
It can be seen that compared to surgical HIPEC, internal medicine HIPEC has a lower incidence of adverse reactions, less trauma, better repeatability, and can be performed multiple times, thus having more advantages. For patients who have not undergone surgery, two-point internal medicine HIPEC with 12F drainage tubes is the least traumatic and more acceptable to patients; for postoperative patients who may have abdominal adhesions, two-point internal medicine HIPEC may cause uneven distribution of perfusion fluid, so four-point internal medicine HIPEC with 8F drainage tubes may be a better choice.
This study compared the differences in flow rate and temperature among four-point surgical HIPEC (22F drainage tubes, inflow at left and right lower abdomen, outflow at left and right upper abdomen), two-point internal medicine HIPEC (12F drainage tubes, inflow at left lower abdomen, outflow at right upper abdomen), and four-point internal medicine HIPEC (8F drainage tubes, inflow at left and right lower abdomen, outflow at left and right upper abdomen) through animal experiments. It demonstrated that although the drainage tubes in internal medicine HIPEC are thinner, they can still achieve the perfusion flow rate and temperature of surgical HIPEC. It is worth noting that this study was an animal experiment with a small sample size. To directly compare the efficacy and safety of surgical and internal medicine HIPEC, further head-to-head clinical trials are needed.
[References]
[1] Wang Hongbo, Wang Pengyuan, Wang Xin, et al. Research progress on operational parameters of hyperthermic intraperitoneal chemotherapy [J]. Modern Oncology Medicine, 2019, 27(21):3930-3933.
WANG HB, WANG PY, WANG X, et al. Research progress of specific parameters of hyperthermic intraperitoneal chemotherapy [J]. Modern Oncology, 2019, 27(21):3930-3933.
[2] DI LEO A, CORVASCE A, WEINDELMAYER J, et al. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in pseudomyxoma peritonei of appendiceal origin: Result of a single centre study [J]. Updates in Surgery, 2020, 72(4):1207-1212.
[3] MORRIS MC, DHAR VK, STEVENSON MA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) for patients at high-risk of peritoneal metastases [J]. Surgical Oncology, 2019, 31:33-37.
[4] FAVIANA P, BOLDRINI L, MUSCO B, et al. Management of peritoneal carcinomatosis with cytoreductive surgery combined with intraperitoneal chemohyperthermia at a novel Italian center [J]. In Vivo (Athens, Greece), 2020, 34(4):2061-2066.
[5] JIAO J, LI C, YU G, et al. Efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) in the management of malignant ascites [J]. World Journal of Surgical Oncology, 2020, 18(1):180.
[6] WONG EYT, TAN GHC, CHIA CSL, et al. Morbidity and mortality of elderly patients following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) [J]. Asia-Pacific Journal of Clinical Oncology, 2018, 14(2):e193-e202.
[7] TSUYOSHI H, INOUE D, KUROKAWA T, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) for gynecological cancer [J]. The Journal of Obstetrics and Gynaecology Research, 2020, 46(9):1661-1671.
[8] LU C, LI L, LUO Z, et al. Clinical efficacy of type-B ultrasound-guided intraperitoneal hyperthermic chemoperfusion combined with systemic chemotherapy in advanced gastric cancer patients with malignant ascites [J]. Neoplasma, 2016, 63(2):299-303.
[9] ORTEGA-DEBALLON P, FACY O, JAMBET S, et al. Which method to deliver hyperthermic intraperitoneal chemotherapy with oxaliplatin: An experimental comparison of open and closed techniques [J]. Annals of Surgical Oncology, 2010, 17(7):1957-1963.
[10] SPRATT JS, ADCOCK RA, MUSKOVIN M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy [J]. Cancer Res, 1980, 40(2):256-260.
[11]YU P, YE Z, DAI G, et al. Neoadjuvant systemic and hyperthermic intraperitoneal chemotherapy combined with cytoreductive surgery for gastric cancer patients with limited peritoneal metastasis: a prospective cohort study [J]. BMC Cancer, 2020, 20(1):1108.
[12]FAN B, BU Z, ZHANG J, et al. Phase II trial of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer after curative surgery [J]. BMC Cancer, 2021, 21(1):216.
[13]BONNOT PE, PIESSEN G, KEPENEKIAN V, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): A propensity score analysis [J]. Journal of Clinical Oncology, 2019, 37(23):2028-2040.
[14]FAGOTTI A, COSTANTINI B, PETRILLO M, et al. Cytoreductive surgery plus HIPEC in platinum-sensitive recurrent ovarian cancer patients: a case-control study on survival in patients with two year follow-up [J]. Gynecol Oncol, 2012, 127(3):502-505.
[15]QUÉNET F, ELIAS D, ROCA L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial [J]. The Lancet Oncology, 2021, 22(2):256-266.
[16]MA YH, YANG J. Comparative analysis of intraperitoneal constant temperature circulatory hyperthermic perfusion chemotherapy and perfusion chemotherapy in the treatment of colorectal cancer [J]. Modern Oncology, 2013, 21(4):825-827.
[17]JIN CY, JING LL. Clinical analysis of hyperthermic intraperitoneal chemotherapy combined with intravenous chemotherapy in the treatment of gastric malignant ascites [J]. Guide of China Medicine, 2017, 15(18):160.
JIN CY, JING LL. Clinical analysis of hyperthermic intraperitoneal chemotherapy combined with intravenous chemotherapy in the treatment of gastric malignant ascites [J]. Guide of China Medicine, 2017, 15(18):160.
[18]CUI SZ. Expert consensus on clinical application of hyperthermic intraperitoneal chemotherapy in China (2019 edition) [J]. National Medical Journal of China, 2020, 100(2):89-96.
CUI SZ. Expert consensus on clinical application of hyperthermic intraperitoneal chemotherapy in China (2019 edition) [J]. National Medical Journal of China, 2020, 100(2):89-96.
[19]LOTTI M, GIULII CAPPONI M, CAMPANATI L, et al. The onset of intra-abdominal adhesions during closed-abdomen hyperthermic intraperitoneal chemotherapy [J]. Journal of Laparoendoscopic & Advanced Surgical Techniques Part A, 2016, 26(12):997-1002.
[20]KUSAMURA S, AZMI N, FUMAGALLI L, et al. Phase II randomized study on tissue distribution and pharmacokinetics of cisplatin according to different levels of intra-abdominal pressure (IAP) during HIPEC (NCT02949791) [J]. European Journal of Surgical Oncology, 2021, 47(1):82-88.
[21]GOURD, MALKA D, TZANIS D, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy [J]. Annals of Surgery, 2013, 257(6):1065-1071.
[22]CHUA TC, YAN TD, SAXENA A, et al. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure: A systematic review of morbidity and mortality [J]. Annals of Surgery, 2009, 249(6):900-907.
[23]LIU L, ZHANG N, MIN J, et al. Retrospective analysis on the safety of 5,759 times of bedside hyperthermic intraperitoneal or intrapleural chemotherapy (HIPEC) [J]. Oncotarget, 2016, 7(16):21570-21578.
(Edited by: Xu Meng)